Thermodynamics – First And Second Law Of Thermodynamics The First Law Of Thermodynamics
The first law of thermodynamics is nothing but energy conservation applied to a thermodynamic system. From the equation W = JH, we know that an amount of work W is spent to produce an equivalent amount of heat H and from H amount of heat, an equivalent amount of work W is obtained.
Hence, heat and mechanical energy (work) are interconvertible. But in practice, when a system takes some amount of heat from its surroundings, it is used up in two ways:
- A part of it increases the internal energy of the system and
- The remaining part is converted into some external work done by the system, i.e., heat absorbed = rise in internal energy + external work done.
This is the first law of thermodynamics.
The first law of thermodynamics is, in essence, the energy conservation law which is applicable to every thermodynamic system together with its surroundings.
Let Ui = initial internal energy of a system,
Q = heat taken by the system from the surroundings,
Uf = final internal energy of the system,
W = external work done by the system.
Here, Uf– Ut = ΔU = change in internal energy. So the first law states that,
Q = \(\left(U_f-U_i\right)+W=\Delta \boldsymbol{U}+\boldsymbol{W}\)….(1)
First Law Of Thermodynamics Equation Class 11 HBSE
In this equation, W and Q are expressed in the same unit.
So Joule’s equivalent is J = 1.
When the internal energy of a system does not change, i.e., Uj-= Up we get, Q= W.
If, on the other hand, W is expressed in joule and Q in calorie, then J ≠ 1. We can then write, W= JQ. (the symbol H for heat is replaced by Q here).
So, Joule’s law of mechanical equivalent of heat is a special case of the first law of thermodynamics (where ΔU =0)
If a small amount of heat dQ changes the internal energy of a system by dU and an external work dW is done, then
dQ = dU + dW……(2)
This is the differential form of the first law of thermodynamics, whereas equation (1) is known as the integral form.
In equations (1) and (2), the convention is:
- Q or dQ is considered positive when heat is absorbed by a system;
- Q or dQ is considered negative when heat is released by a system.
First Law Of Thermodynamics Class 11 HBSE Notes
Significance of the first law of thermodynamics: From the first law of thermodynamics, it is known that mechanical energy can be converted into heat and vice versa. Certain amount of heat is required to do a certain amount of work. On the other hand, a certain amount of work has to be done to generate a certain amount of heat.
- The first law of thermodynamics indicates a new property of a body internal energy. In different thermodynamic processes, if we take only work and heat as the two manifestations of energy, the conservation of energy principle is violated.
- However, the body in every state has some internal energy. In every process, if we consider the change of this internal energy along with heat and work, energy remains conserved in all cases.
- This internal energy (U) is a state function. During the transformation from one state of a body to another, the change of internal energy does not depend on the intermediate path. In thermodynamics, there is no need to identify the source of internal energy.
- But it is seen from the kinetic theory of gases that, the kinetic energy and the potential energy due of to translational, rotational, and vibrational motions of the molecules of a body are the sources of its internal energy.
The perpetual motion of the first kind: it is impossible to get work without dissipation of energy. So, it is impossible to invent a machine that can work indefinitely without any supply of energy. If a machine can work indefinitely without any energy input, its motion is called the perpetual motion of the first kind.
But the first law of thermodynamics, which expresses the law of conservation of energy, states that this is impossible. So, perpetual motion of the first kind does not exist in nature.
Limitations Of First Law Of Thermodynamics Class 11 HBSE
Origin of internal energy: For complete conversion between W and Q, we have W = Q. Then from the first law, Uf – Ui = 0 or, Uf = Ui i.e., the internal energy of a system does not change.
- But complete conversion between W and Q is an ideal case, and has some natural restrictions. Thus, directly from the law of conservation of energy, we get the existence of a new thermodynamic property — the internal energy U of a system.
- For example, when a system takes some heat from outside but does no work (W = 0), we have Q = Uf– Ui, or heat absorbed = increase in internal energy.
- So, the effect of heat on a system, doing no work, is a change in its internal energy. These effects are rise in temperature, melting of a solid, vaporization of a liquid, etc. In each of these examples, the change in temperature of the system or the latent heat is directly related to the change in its internal energy.
When the motion of the molecules in a system is considered, we get a clear picture of the internal energy of a system. But thermodynamics does not discuss molecular motions that will be dealt with in kinetic theory.
First Law Of Thermodynamics Derivation Class 11 HBSE
Internal energy of a gas: Thermal condition of a fixed mass of gas is determined by three quantities temperature, pressure and volume. But internal energy of a gas does not depend on all of these three quantities. It depends only on its temperature in some cases.
- For example, there is no change in internal energy if the pressure or volume of a monatomic ideal gas of a particular mass changes at constant temperature. If we know the rise in temperature of this type of gas, we can determine the increase in internal energy.
- But the change in internal energy does not always depend on the change in temperature. If a gas undergoes a phase change, there is no change in temperature, but its- internal energy changes.
Statement Of First Law Of Thermodynamics Class 11 HBSE
Limitations of the first law of thermodynamics: This law expresses the law of energy conservation. That is, it says that energy cannot be created or destroyed it can only be converted from one form to another. But it cannot predict anything about the direction of natural processes. It fails to explain why
- Heat can flow only from higher to lower temperature and never from lower to higher temperature
- Some amount of work can entirely be converted into heat, but complete conversion of heat into work never occurs in nature.
So, the maximum heat or work available from a certain amount of work or heat, respectively, cannot be determined from the first law of thermodynamics. These limitations are overcome by the formulation of another law, the second law of thermodynamics.
First Law Of Thermodynamics And Internal Energy Class 11 HBSE
Thermodynamics – First And Second Law Of Thermodynamics The First Law Of Thermodynamics Numerical Examples
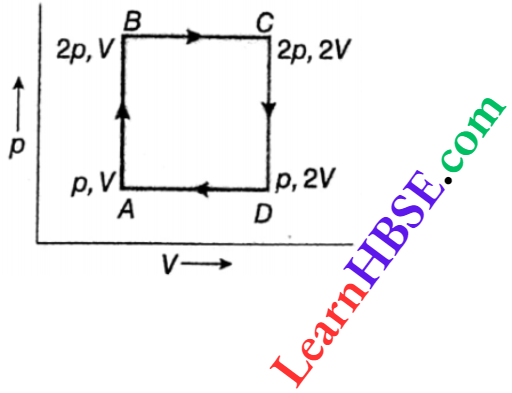
Example 1. An ideal monatomic gas goes through a cyclic process ABCDA, as shown. Find out the work done and heat supplied in this cyclic process.
Applications Of First Law Of Thermodynamics Class 11 HBSE
Solution:
Work done in the cycle ABCD = area of ABCD
= AB-BC = (2p-p)(2V-V) = pV
Here, the initial state A = the final state A.
So, the change in internal energy, Uf – Ui = UA – UA =0
Then, Uf– Ui = Q-W or, Q = (Uf – Ui) + W= 0 + pV = pV
∴ Heat supplied in the cyclic process = pV.
First Law Of Thermodynamics Formula Class 11 HBSE
Example 2. The volume of 1 g of water (1 cm³) becomes 1671 cm³ on being converted to steam at standard atmosphere pressure. Find out the work done and rise in internal energy. Given, the latent heat of vaporization of water = 540 cal · g-1 the standard atmosphere pressure = 1.013 x 105 N · m-2.
Solution:
W = \(\int_{V_1}^{V_2} p d V=p \int_{V_1}^{V_2} d V=p\left(V_2-V_1\right)\)
Here, \(V_2-V_1=1671-1\)
= \(1670 \mathrm{~cm}^3\)
= \(1670 \times 10^{-6} \mathrm{~m}^3=1.67 \times 10^{-3} \mathrm{~m}^3\)
First Law Of Thermodynamics Numerical Problems Class 11 HBSE
∴ W = \(\left(1.013 \times 10^5\right) \times\left(1.67 \times 10^{-3}\right) \)
= 169.17 J
∴ \(U_2-U_1=Q-W=540 \times 4.2-169.17\)
(\(540 \mathrm{cal}=540 \times 4.21\))
= 2099 J.
