Question 21. Three samples, A, B, and C of gas initially have the same volume. The volume of each sample is doubled by the following processes:
- An isothermal process on a,
- Adiabatic process on b, and
- Isobaric process on c. In which process will the work done be highest?
Answer:
The processes on the three samples are shown on a pV diagram. Two vertical lines X and Y denote the volumes V and 2V. In this diagram, the area under a curve represents the work done.

Clearly, this area is the largest for the isobaric process on sample C. So the work done in this process will be the highest.
First Law Of Thermodynamics Class 11 Derivation HBSE
Question 22. An ideal gas of mass is mistaken from state A to state B in three different processes 1,2 and 3. If the work done in the three processes are W1, W2, and W3, arrange them in descending order.
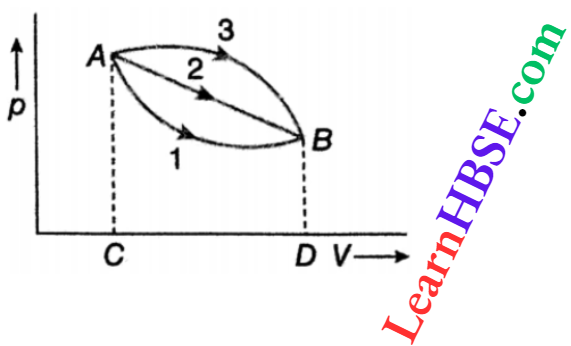
Answer:
The area under a curve on a pV diagram represents the work done in the corresponding process.
Area A3BDCA > area A2BDCA > area AIBDCA.
So, W3 >W2>W1.
First And Second Law Of Thermodynamics Class 11 HBSE Notes
Question 24. In a process on an ideal gas, dW = 0 and dQ< 0. Show that the temperature of the gas will fall.
Answer:
Given
In a process on an ideal gas, dW = 0 and dQ< 0
In an adiabatic process, Q = 0
From the first law of thermodynamics, \(U_f-U_i=Q-W=0-W=-W \text { or, } W=U_{i^{-}}-U_f \text {. }\)
In a compression, W is negative.
So, Ui – Uf <0 or, Ui < Uf. This means that the internal energy increases. The temperature of an ideal gas will also increase with the increase in internal energy.
Relation Between First And Second Law Of Thermodynamics Class 11
Question 25. When the tyre of a bicycle or motor car bursts suddenly, cool air comes out from the tube. Explain why.
Answer:
When a tube bursts, the air in it expands very rapidly. A very rapid process is essentially an adiabatic process. We know that the temperature of the gas falls in such an adiabatic expansion. So, cool air comes out from a burst tube.
Second Law Of Thermodynamics Class 11 Physics HBSE
Question 26. The molar specific heats Cp and Cv of an ideal gas are expressed in the unit of cal • mol-1 • °C-1. But R is expressed in J • mol-1 • °C-1. Write down the relation between Cp and Cv.
Answer:
Given
The molar specific heats Cp and Cv of an ideal gas are expressed in the unit of cal • mol-1 • °C-1. But R is expressed in J • mol-1 • °C-1.
R J · mol-1 · °C-1 = R/J ca l · mol-1 · °C-1.
So the relation Cp – Cv = R is to be written as Cp – Cv = R/J, where J = Joule’s equivalent.
Question 27. Cp – Cv = a for hydrogen gas and Cp – Cv= b for oxygen gas. What is the relation between a and h?
Answer:
Given
Cp – Cv = a for hydrogen gas and Cp – Cv= b for oxygen gas
The relation between a and h
Cp and Cv are molar-specific heats. As hydrogen and oxygen gases satisfy the properties of an ideal gas, Cp -Cv= R for both the gasses. So, a = b.
Question 28. An ideal gas undergoes four processes: isochoric, isobaric, isothermal, and adiabatic. In which process is
- The change in internal energy is zero,
- The work done zero and
- Is the heat exchange zero?
Answer:
- Internal energy of an ideal gas does not change when the temperature is constant. So the corresponding process is an isothermal process.
- Work done is zero when the volume remains constant. So the corresponding process is an isochoric process.
- Heat exchange with the surroundings is zero in an adiabatic process.
First And Second Law Of Thermodynamics Examples Class 11
Question 29. A system goes from state A to state B in two different processes 1 and 2. What is the relation between the respective changes \(\Delta U_{\mathrm{1}} \text { and } \Delta U_{\mathrm{2}}\) of internal energy?
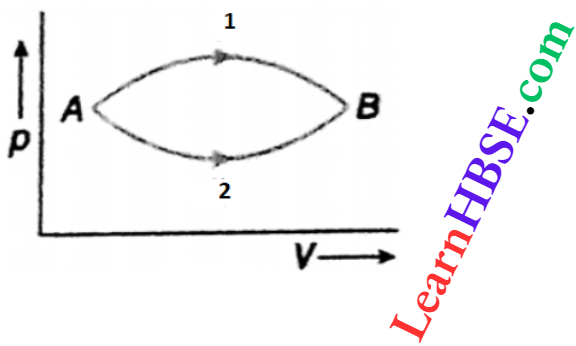
Answer:
The initial and the final states are the same for both processes. Internal energy is a function of state. The value of ΔU is not path-dependent
So, ΔU = UB – UA and ΔU= UB – UA
∴ ΔU1 = ΔU2
Question 30. One should not try to cool a kitchen by leaving the refrigerator door open. Explain why.
Answer:
- The refrigerator releases heat by absorbing it from the food kept inside it. If the door of a refrigerator is kept open, the refrigerator absorbs heat from the kitchen and releases it in the kitchen itself.
- For that, no temperature change should occur. But during this process, the compressor of the refrigerator converts some electric energy supplied to it into heat energy. As a result, the kitchen temperature tends to increase slightly.
Question 31. The volume, pressure, and temperature of some amount of gas are V, p, and T, respectively. A partition divides the gas into two equal parts. What will be the values of the quantities in each part?
Answer:
Given
The volume, pressure, and temperature of some amount of gas are V, p, and T, respectively. A partition divides the gas into two equal parts.
The volume V is an extensive property. So the volume of each half would be But the pressure p and the temperature T are intensive properties. So they remain unaltered, i.e., the values of these quantities in each half of the gas will be p and T.
First And Second Law Of Thermodynamics Formula Class 11
Question 32. Show that the adiabatic bulk modulus of an ideal gas is equal to the product of the pressure of the gas and the ratio of its two specific heats (γ).
Answer:
In case of adiabatic change of some fixed mass of ideal gas, pVγ = constant,
where, \(\gamma=\frac{\text { specific heat of the gas at constant pressure }}{\text { specific heat of the gas at constant volume }}\)
Differentiating the above relation we get, \(V^\gamma d p+p \gamma V^{\gamma-1} d V=0\)
or, \(V^\gamma d p+\gamma p \frac{V \gamma}{V} d V=0\)
or, \(\frac{d p}{p}=-\gamma \frac{d V}{V}\)
∴ \(K_s=\frac{d p}{-\frac{d V}{V}}=\gamma p\)
[where Ks is the adiabatic bulk modulus of the gas]
So, the adiabatic bulk modulus of an ideal gas is equal to the product of the pressure of the gas and the ratio of its two specific heats (γ).
First And Second Law Of Thermodynamics Conclusion
A body or a part of a body or a combination of a number of bodies whose properties are being studied is usually called a thermodynamic system. In general, such a system exchanges energy and mass with its surroundings.
Thermodynamic properties are of two types:
- Extensive properties (examples: mass, volume, internal energy, entropy, etc.) and
- Intensive properties (examples: pressure, temperature, density, specific heat, coefficient of expansion, modulus of elasticity, etc.)
Joules Law In Thermodynamics: If some amount of work is entirely converted into heat, the work done and the heat produced are proportional to each other.
The amount of work done to produce unit amount of heat is called the mechanical equivalent of heat or Joule’s equivalent (J).
The first law of thermodynamics: When a system absorbs some amount of heat from the surroundings,
- A part of it increases the internal energy of the system and
- The remaining part is converted into some external work done by the system.
The internal energy of a particular amount of ideal gas depends on its temperature only. This property is approximately true for real gases also.
Applications Of First And Second Law Of Thermodynamics Class 11
Heat absorbed by unit mass of a substance to raise the temperature by one degree, when
- The volume is kept constant and
- The pressure is kept constant, are called the specific heat at constant volume (cv) and the specific heat at constant pressure (cp) respectively.
- When 1 mol of a substance is taken, instead of unit mass, the corresponding quantities are called the molar specific heat at constant volume (Cv) and the molar specific heat at constant pressure (Cp).
- A process in which the temperature of a system remains constant is called an isothermal process. The changes in volume and pressure in an isothermal process are called isothermal changes.
- A process in which no heat is exchanged between a system and its surroundings is called an adiabatic process. The changes in volume, pressure, and temperature in an adiabatic process are called adiabatic changes.
The second law of thermodynamics:
- Clausius’s statement: No self-acting machine can transfer heat from a lower to a higher temperature.
- Kelvin-Planck statement: No self-acting machine can convert some amount of heat entirely into work.
A reversible process is one that can be reversed and the heat exchange and the work done in each infinitesimal step in the reverse process are exactly equal and opposite to those in the forward process.
- A process that does not satisfy these conditions is an irreversible process.
- Every process in nature occurs in such a direction that the total entropy of the universe increases. This is known as the principle of increase in entropy. It is the most general statement of the second law of thermodynamics.
First Law Of Thermodynamics Equation Class 11 HBSE
Each of the three laws of thermodynamics defines one important property of all thermodynamic systems
- Zeroth law: Temperature (T),
- First law: Internal energy (U),
- Second law: Entropy (S)
Heat engine is a mechanical device which converts heat into work. The efficiency of heat engine is defined as the fraction of total heat taken from the source which is converted into work.
- A mechanical device that transfers heat from a colder to a hotter place is called a refrigerator.
- An ideal heat engine and an ideal refrigerator do not exist in nature.
- A cycle enclosed by four reversible processes, two iso-thermals and two adiabatics, is called a Carnot cycle.
- A clockwise Carnot cycle acts as a heat engine which is enclosed by two reversible isothermal and two revers¬ible adiabatic processes. This is called a Carnot engine.
Entropy And Second Law Of Thermodynamics Class 11 Physics
Carnot showed that
- All reversible engines working between the same two temperatures have the same efficiency and
- No engine working between two given temperatures can be more efficient than a reversible engine working between the same two temperatures. This is Carnot’s theorem.
