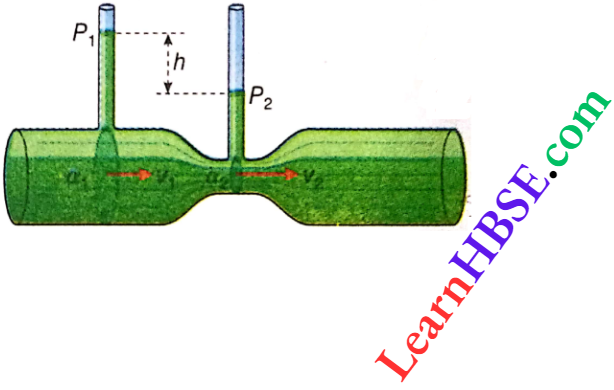
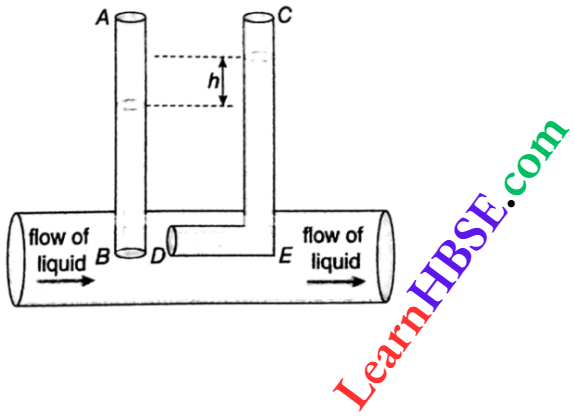
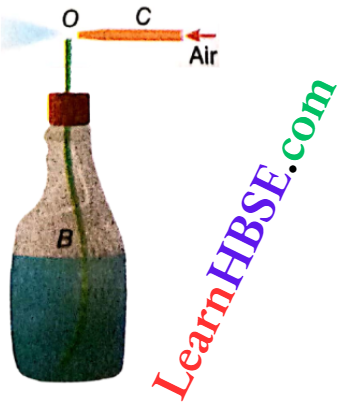
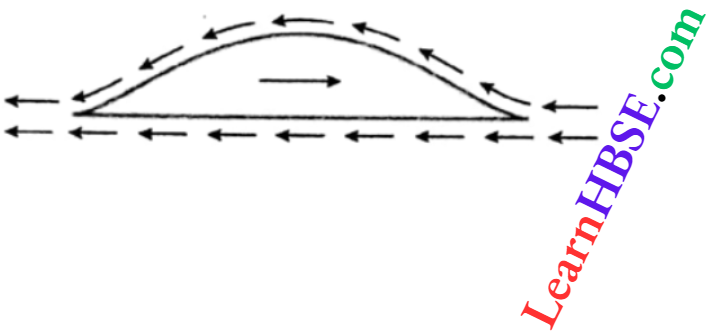
Expansion Of Gases Relation Between Pressure, Temperature And Density
Relation Between Pressure, Temperature And Density:
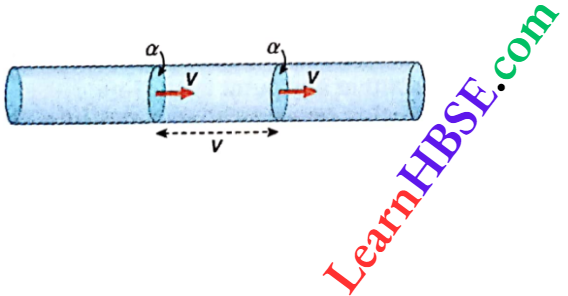
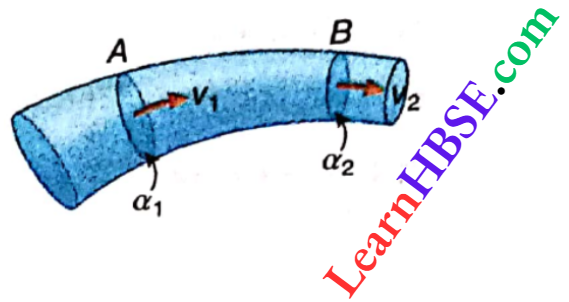
Let an ideal gas is taken of molecular weight M (say).
For m1 mass of the gas, the volume and density be V1 and ρ1, respectively at a temperature T1 K. When its mass is m2 the volume and density are V2 and ρ2 respectively at T2 K.
∴ \(V_1=\frac{m_1}{\rho_1} \text { and } V_2=\frac{m_2}{\rho_2}\)
If p1 and p2 are the pressures at those temperatures, then from the equation pV = nRT = m/M RT we have,
⇒ \(\frac{p_1 V_1}{m_1 T_1}=\frac{p_2 V_2}{m_2 T_2}\) [since for a particular gas M is constant]
or, \(\frac{p_1}{\rho_1 T_1}=\frac{p_2}{\rho_2 T_2}\)
∴ \(\frac{p}{\rho T}=\) constant
1. If the pressure is a constant, \(\rho_1 T_1=\rho_2 T_2 \text { i.e., } \rho T=\text { constant or, } \rho \propto \frac{1}{T}\)
i.e., at constant pressure, the density of a gas is inversely proportional to Its absolute temperature.
2. If the temperature is a constant,
\(\frac{p_1}{\rho_1}=\frac{p_2}{\rho_2} \text { or, } \frac{p}{\rho}=\text { constant or, } \rho \propto p\)
i.e., at a constant temperature, the density of a gas is directly proportional to its pressure.
Expansion Of Gases Relation Between Pressure, Temperature And Density Numerical Examples
Example 1. Temperature and pressure on top of a hill are 7°C and 70 cmHg, and the corresponding values at its base are 27°C and 76 cmHg. Compare the densities of air at the top and the base of the hill.
Solution:
Given
Temperature and pressure on top of a hill are 7°C and 70 cmHg, and the corresponding values at its base are 27°C and 76 cmHg.
We use the relation \(\frac{p_1}{\rho_1 T_1}=\frac{p_2}{\rho_2 T_2}\)
Here, p1 = 70 cmHg, T1 = 273 + 7 = 280 K, p2 = 76 cmHg and T2 = 273 + 27 = 300 K.
⇒ \(\frac{70}{\rho_1 \times 280}=\frac{76}{\rho_2 \times 300} \text { or, } \frac{\rho_1}{\rho_2}=\frac{300 \times 70}{76 \times 280}=\frac{75}{76} \text {. }\)
Relation Between Pressure And Density In Fluids
Example 2. Density of argon at 27°C and 76 cmHg pressure Is 1.6 g · L-1. An electric bulb of volume 200 cm3 is filled with argon. The pressure of the gas inside the bulb is 75 cmHg and the average temperature is 127°C. Find the mass of argon gas in the bulb.
Solution:
Given
Density of argon at 27°C and 76 cmHg pressure Is 1.6 g · L-1. An electric bulb of volume 200 cm3 is filled with argon. The pressure of the gas inside the bulb is 75 cmHg and the average temperature is 127°C.
Let the density of the gas in the bulb at a pressure of 75 cmHg and at 127°C =ρ2
Here, p1 = 76 cmHg, ρ1 = 1.6 g · L-1,
T1 = 273 + 27 = 300 K
p2 = 75 cmHg,
T2 = 273 + 127 = 400 K
From the relation \(\frac{p_1}{\rho_1 T_1}=\frac{p_2}{\rho_2 T_2}\) we get,
⇒ \(\frac{76}{1.6 \times 300}=\frac{75}{\rho_2 \times 400}\)
∴ \(\rho_2=\frac{75 \times 1.6 \times 300}{76 \times 400}=1.184 \mathrm{~g} \cdot \mathrm{L}^{-1}\)
∴ Mass of argon gas of volume 200cm³
= \(\frac{200}{1000} \times 1.184=0.2368 \mathrm{~g} .\)
Example 3. At a place, air pressure is 75 cmHg and the temperature is 27°C. At another place, the respective values are 70 cmHg and 17°C. Compare the densities of air in the two places.
Solution:
Given
At a place, air pressure is 75 cmHg and the temperature is 27°C. At another place, the respective values are 70 cmHg and 17°C.
We have, \(\frac{p_1}{\rho_1 T_1}=\frac{p_2}{\rho_2 T_2}\)
Given, p1 = 75 cmHg, T1 = 273 + 27 = 300 K, p2 = 70 cmHg
and T2 = 273 + 17 = 290 K
∴ \(\frac{75}{\rho_1 \times 300}=\frac{70}{\rho_2 \times 290} \text { or, } \frac{\rho_1}{\rho_2}=\frac{75 \times 290}{300 \times 70}=\frac{29}{28}\)
∴ \(\rho_1: \rho_2=29: 28\)
Pressure And Density Relation In Liquids
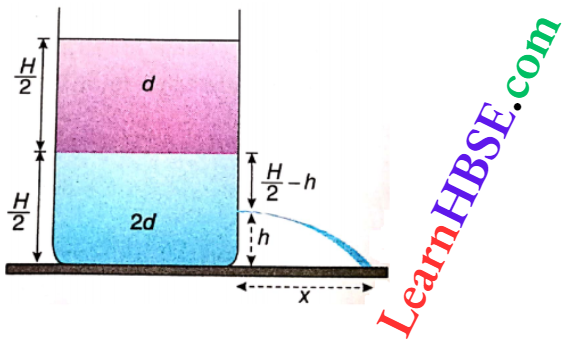
Example 4. When an air bubble rises from the bottom of a lake to the upper surface, its diameter increases from 1 mm to 2 mm. If the atmospheric pressure is 76 cmHg, calculate the depth of the lake. Density of mercury is 13.6 g · cm-3.
Solution:
Given
When an air bubble rises from the bottom of a lake to the upper surface, its diameter increases from 1 mm to 2 mm. If the atmospheric pressure is 76 cmHg,
Density of mercury is 13.6 g · cm-3.
Let the depth of the lake be h cm.
Pressure on the air bubble, at the bottom of the lake, p1 = atmospheric pressure + pressure of water at depth h = (76 x 13.6 x 981 + h x 1 x 981) dyn · cm-2, and pressure at the surface of the lake, p2 = atmospheric pressure = 76 x 13.6 x 981 dyn · cm-2
Volumes of the bubble: at the bottom, \(V_1=\frac{4}{3} \pi\left(\frac{1}{20}\right)^3 \mathrm{~cm}^3\)
at the top, \(V_2=\frac{4}{3} \pi\left(\frac{1}{10}\right)^3 \mathrm{~cm}^3\)
Using Boyle’s law: p1 V1 = p2 V2, we get,
(h x 1 x 981 + 76 x 13.6 x 981) x \(\times \frac{4}{3} \pi\left(\frac{1}{20}\right)^3\)
= 76 x 13.6 x 981 x \(\times \frac{4}{3} \pi\left(\frac{1}{10}\right)^3\)
or, (h x 76 x 13.6) x 1/8 = 76 x 13.6
or, h = 76 x 13.6 x 8 – 76 x 13.6
∴ The lake is 7235.2 cm or 72.352 m deep
Difference Between Pressure And Density In Physics
Example 5. An electronic vacuum tube is constructed and sealed at 27°C and 1.2 x 10-6 cm Hg pressure. The tube has a volume of 100 cm³. Calculate the number of gas molecules left In the tube. Avogadro number is 6.02 x 1023, and the gas occupies a volume of 22.4 litres at STP.
Solution:
Given
An electronic vacuum tube is constructed and sealed at 27°C and 1.2 x 10-6 cm Hg pressure. The tube has a volume of 100 cm³.
Avogadro number is 6.02 x 1023, and the gas occupies a volume of 22.4 litres at STP
Molar density at STP = \(\frac{6.02 \times 10^{23}}{22.4 \times 10^3} \text { molecules } \cdot \mathrm{cm}^{-3}\)
Let the number of molecules left in the tube, after sealing, be n.
From gas equation we have, \(\frac{p_1}{\rho_1 T_1}=\frac{p_2}{\rho_2 T_2}\)
Here, \(p_1=1.2 \times 10^{-6} \mathrm{cmHg}\)
⇒ \(\rho_1=\frac{n}{100} \text { molecules } \cdot \mathrm{cm}^{-3}\)
⇒ \(T_1=273+27=300 \mathrm{~K} \text {, }\)
⇒ \(p_2=76 \mathrm{cmHg} \text {, }\)
⇒ \(\rho_2=\frac{6.02 \times 10^{23}}{22.4 \times 10^3} \text { molecules } \cdot \mathrm{cm}^{-3}\)
and \(T_2=273 \mathrm{~K}\)
∴ \(\frac{1.2 \times 10^{-6}}{\frac{n}{100} \times 300}=\frac{76}{\frac{6.02 \times 10^{23}}{22.4 \times 10^3} \times 273}\)
i.e., n = \(\frac{1.2 \times 10^{-6} \times 100 \times 6.02 \times 10^{23} \times 273}{300 \times 76 \times 22.4 \times 10^3}\)
= \(3.86 \times 10^{13} \text {. }\)
Example 6. While constructing a bulb of volume 250 cm³, it is sealed at 27° C temperature and 10-3 mmHg pres-sure. Find the number of gas molecules in the bulb. Avogadro number = 6.0 x 1023.
Solution:
Given
While constructing a bulb of volume 250 cm³, it is sealed at 27° C temperature and 10-3 mmHg pres-sure.
Volume of air inside the bulb, V1 = 250 cm³; pressure of air, p1 = 10-3 mmHg = 10-4 cmHg; temperature T1 = 27 + 273 = 300 K.
Let at STP the volume of air = V2 cm³; p2 = 76 cmHg; T2 = 273 K
∴ Using \(\frac{p_1 V_1}{T_1}=\frac{p_2 V_2}{T_2}\) we get,
⇒ \(V_2=\frac{p_1 V_1 T_2}{T_1 p_2}=\frac{10^{-4} \times 250 \times 273}{300 \times 76}=29.934 \times 10^{-5} \mathrm{~cm}^3\)
At STP, number of molecules in air of volume 22400 cm³ = 6 x 1023.
So, that in volume 29.934 x 10-5 cm3
= \(\frac{6 \times 10^{23}}{22400} \times 29.934 \times 10^{-5}=8.018 \times 10^{15}\)
Atmospheric Pressure And Air Density Relationship
Example 7. Two containers of volume 5 L and 3 L contain air at 3 standard atmospheres and 7 standard atmospheres respectively. The containers are now connected by a short narrow tube. What will be the common pressure in both containers?
Solution:
Given
Two containers of volume 5 L and 3 L contain air at 3 standard atmospheres and 7 standard atmospheres respectively. The containers are now connected by a short narrow tube.
Suppose the containers have n1 and n2 number of moles of the gas.
∴ 5×3 = n1RT….(1)
and 3×7 = n2RT…(2)
Therefore, 15 + 21 = (n1 + n2)RT [adding (1) and (2)]
or, (n1 + n2)RT= 36
At constant temperature, let the common pressure attained be p.
At that common pressure p, p(5 + 3) = (n1 + n2)RT = 36
∴ p = 36/8 = 4.5 standard atmospheres.
Example 8. Two bulbs of equal volume are connected by a narrow tube of negligible volume and filled with a gas at STP. If one of the bulbs is kept in melting ice and the other in a water bath at 62°C, what will be the new pressure of the gas?
Solution:
Given
Two bulbs of equal volume are connected by a narrow tube of negligible volume and filled with a gas at STP. If one of the bulbs is kept in melting ice and the other in a water bath at 62°C
Let volume of each bulb =Vcm³ and each contains n number of moles of the gas.
∴ Total number of moles contained in the bulb initially
= \(2 n=\frac{76 \times 2 V}{R \times 273} \quad[\text { from } p V=n R T]\)….(1)
Let the final pressure in both the bulbs = p.
Number of moles in one of the bulbs = \(\frac{p V}{R \times 273}\)
and that in the other bulb = \(\frac{p V}{T(273+62)}=\frac{p V}{R \times 335}\)
∴ Total number of moles contained in the bulbs = \(\frac{p V}{R \times 273}+\frac{p V}{R \times 335}=\frac{p V}{R}\left(\frac{1}{273}+\frac{1}{335}\right)\)…(2)
From (1) and (2) we get, \(\frac{76 \times 2 V}{R \times 273}=\frac{p V}{R}\left[\frac{1}{273}+\frac{1}{335}\right]\)
or, \(\frac{p V}{R}(0.0037+0.0030)=\frac{76 \times V \times 2}{R \times 273}\)
or, \(p \times 0.0067=0.5568 or, p=\frac{0.5568}{0.0066}=83.10 \mathrm{cmHg}\).
Bulk Modulus And Relation Between Pressure And Density
Example 9. An air bubble rises from the bottom of a lake to its upper surface. The diameters of the bubble at the bottom and the surface are 3.6 mm and 4 mm respectively. Depth of the lake is 2.5 m and the temperature at the upper surface is 40°C. Find the temperature at the bottom of the lake. Ignore the change in density of water with height. (Atmospheric pressure = 76 cmHg and g = 980cm · s-2)
Solution:
Given
An air bubble rises from the bottom of a lake to its upper surface. The diameters of the bubble at the bottom and the surface are 3.6 mm and 4 mm respectively. Depth of the lake is 2.5 m and the temperature at the upper surface is 40°C.
Pressure at the bottom of the lake p1 =76x 13.6×980 + 250x 1×980
= (76 x 13.6 + 250) x 980 dyn · cm-2
Volume of the air bubble at the bottom \(V_1=\frac{4}{3} \pi(0.18)^3 \mathrm{~cm}^3\)
Let the temperature at the bottom of the lake = T1
Now, at the surface, pressure p2 = 76 x 13.6 x 980 dyn · cm-2
volume of the air bubble \(V_2=\frac{4}{3} \pi(0.2)^3 \mathrm{~cm}^3 \text {; }\); temperature T2 = 273 + 40 = 313 K
Now using \(\frac{p_1 V_1}{T_1}=\frac{p_2 V_2}{T_2}\), we get
⇒ \(\frac{(76 \times 13.6+250) \times 980 \times \frac{4}{3} \pi(0.18)^3}{T_1 . \quad}=\frac{76 \times 13.6 \times 980 \times \frac{4}{3} \pi(0.2)^3}{313}\)
or, \(T_1=283.37 \mathrm{~K}=283.37-273^{\circ} \mathrm{C}=10.37^{\circ} \mathrm{C}\).
Example 10. A balloon at STP can lift a total mass of 175 kg attached with it When the barometer reads 50 cmHg and the temperature becomes -10°C at an upper point to where the balloon rises, find the maximum mass that can be lifted. Consider the volume of the balloon to be a constant.
Solution:
Given
A balloon at STP can lift a total mass of 175 kg attached with it When the barometer reads 50 cmHg and the temperature becomes -10°C at an upper point to where the balloon rises
The change in lifting capacity is due to the change in the upthrust, as the density of air changes at higher altitude due to the change in temperature and pressure.
Let V = volume of the balloon, ρ1 = density at STR ρ2 = density at 50 cmHg and -10°C; M = mass it can carry at the given altitude.
Hence, from Archimedes’ principle, \(V \rho_1=175 \mathrm{~kg} \text { and } V \rho_2=M \mathrm{~kg}\)
Now, \(\frac{p_1}{\rho_1 T_1}=\frac{p_2}{\rho_2 T_2} or, \frac{p_1}{V \rho_1 T_1}=\frac{p_2}{V \rho_2 T_2}\)
Substituting the corresponding values, we get, \(\frac{76}{175 \times 273}=\frac{50}{M \times 263}\)
or, M = \(\frac{175 \times 273 \times 50}{263 \times 76}=119.5 \mathrm{~kg}\).
Pressure Vs Density Graph Explanation
Example 11. A chamber contains a mass m1 of a gas at pressure p1. A second chamber contains a mass m2 of the same gas at pressure p2. If the two chambers are now connected, what will be the pressure of the gas mixture?
Solution:
Given
A chamber contains a mass m1 of a gas at pressure p1. A second chamber contains a mass m2 of the same gas at pressure p2. If the two chambers are now connected,
Let V1 = volume of the 1st chamber, V2 = volume of the 2nd chamber,
M = molecular weight of the gas, p = final pressure of the gas mixture,
T = constant temperature before and after mixing of the gases.
We use the relation \(p V=n R T=\frac{m}{M} R T\)
∴ For the 1st chamber, \(p_1 V_1=\frac{m_1}{M} R T\)
and for the 2nd chamber, \(p_2 V_2=\frac{m_2}{M} R T\)
∴ \(p_1 V_1+p_2 V_2=\left(m_1+m_2\right) \frac{R T}{M}\)
Using the relation for the gas mixture, we have \(p\left(V_1+V_2\right)=\left(m_1+m_2\right) \frac{R T}{M}\)
∴ \(p\left(V_1+V_2\right)=p_1 V_1+p_2 V_2 \text { or, } p=\frac{p_1 V_1+p_2 V_2}{V_1+V_2}\)
Now, \(V_1=\frac{m_1}{p_1} \frac{R T}{M} and V_2=\frac{m_2}{p_2} \frac{R T}{M}\).
So, \(V_1+V_2=\left(\frac{m_1}{p_1}+\frac{m_2}{p_2}\right) \frac{R T}{M}=\frac{p_2 m_1+p_1 m_2}{p_1 p_2} \frac{R T}{M}\)
∴ p = \(\left(m_1+m_2\right) \frac{R T}{M} \cdot \frac{p_1 p_2}{p_2 m_1+p_1 m_2} \cdot \frac{M}{R T}\)
= \(\frac{p_1 p_2\left(m_1+m_2\right)}{p_2 m_1+p_1 m_2} .\)
Example 12. A 100 cm long glass capillary tube, closed at both ends, has a mercury thread of length 10 cm. When the tube is horizontal, the mercury thread stays at the middle of the tube with air columns of equal length on either side, at 76 cmHg pressure and 27°C. Now, the temperature of one side is changed to 0°C, and of the other side to 127°C. Find the length and the pressure of the air column kept at 0°C. Neglect expansions of glass and mercury.
Solution:
Given
A 100 cm long glass capillary tube, closed at both ends, has a mercury thread of length 10 cm. When the tube is horizontal, the mercury thread stays at the middle of the tube with air columns of equal length on either side, at 76 cmHg pressure and 27°C. Now, the temperature of one side is changed to 0°C, and of the other side to 127°C.
Let the area of the cross-section of the capillary be α cm².
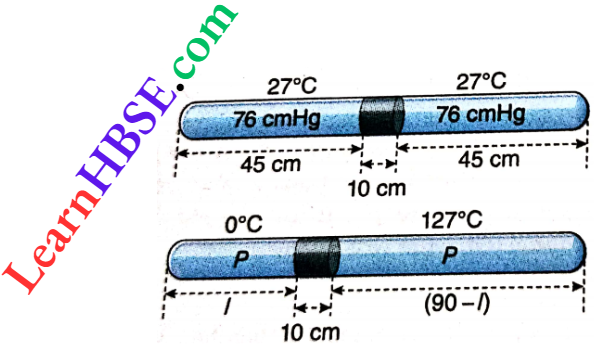
In the first case, the volume of air on either side of the mercury thread in the tube, V1 = 45α cm³; temperature,
T1 = 273 + 27 = 300 K; pressure of air, p1 = 76 cmHg.
In the second case, let the left side of the Hg thread inside the tube be at 0°C or 273 K and the other side be at 127°C or 400 K.
Let the pressure of the confined air on each side = p cm.
Let l = length of the air column at 0°C; (90 -l) = length of the other air column at 127°C.
Using the equation of state, \(\frac{p_1 V_1}{T_1}=\frac{p_2 V_2}{T_2}, \frac{76 \times 45 \alpha}{300}=\frac{p l \alpha}{273}\)…(1)
and \(\frac{76 \times 45 \alpha}{300}=\frac{p(90-l) \alpha}{400}\)…(2)
From (1) and (2) we get, \(\frac{p \times l \times \alpha}{273}=\frac{p(90-l) \alpha}{400}\)
or, \(400 l=273(90-l) or, 673 l=273 \times 90\)
∴ l = \(\frac{90 \times 273}{673}=36.5 \mathrm{~cm} \text {. }\)
From equation (1), \(\frac{76 \times 45 \alpha}{300}=\frac{p \times 36.5 \alpha}{273} \text { or, } p=85.3 \mathrm{cmHg}\)
∴ The length of the air column at 0°C is 36.5 cm and its pressure is 85.3 cmHg.
Pressure And Density Formula In Fluid Mechanics
Example 13. Two heat proof containers of volumes IL and 3L are connected by a tube. Keeping the valve attached to the tube dosed, the 1st container is filled with nitrogen at 0°C and at 0.5 standard atmosphere pressure, and the 2nd container with argon at 100°C and at 1.5 standard atmosphere pressure. The temperature of the gas mixture becomes 79 °C when the valve is opened. Find the pressure of the gas mixture.
Solution:
Given
Two heat proof containers of volumes IL and 3L are connected by a tube. Keeping the valve attached to the tube dosed, the 1st container is filled with nitrogen at 0°C and at 0.5 standard atmosphere pressure, and the 2nd container with argon at 100°C and at 1.5 standard atmosphere pressure. The temperature of the gas mixture becomes 79 °C when the valve is opened.
In the 1st container, pressure of nitrogen (p1) = 0.5 standard atmosphere, volume (V1) = 1 L, temperature (T1) = 0 + 273 = 273 K;
and in the 2nd container, pressure of argon (p2) = 1.5 standard atmosphere, volume (V2) = 3 L, temperature (T2) = 100 + 273 = 373 K
When the valve is opened, let the pressure of the gas mixture be p volume, V = 4L and temperature, T = 273 + 79 = 352 K
As \(\frac{p V}{T}=n R\),
we have, \(n_1 R+n_2 R=\left(n_1+n_2\right) R\)
or, \(\frac{p_1 V_1}{T_1}+\frac{p_2 V_2}{T_2}=\frac{p V}{T}\)
or, \(\frac{0.5 \times 1}{273}+\frac{1.5 \times 3}{373}=\frac{p \times 4}{352} \)
or, \(\frac{p}{88}=0.0018+0.0120\)
∴ p =0.0138×88 = 1.2144 standard atmosphere.
Example 14. Two glass bulbs of volumes 3 L and 1 L are connected by a narrow tube. The system is filled with air at 30°C temperature and at 76 cmHg pressure. Now the bulb of volume 3 L is immersed in water vapour at temperature 100°C while the other bulb is kept at 30°C. Find the air pressures in the two bulbs. Neglect the volume expansion of the 3 L bulb.
Solution:
Given
Two glass bulbs of volumes 3 L and 1 L are connected by a narrow tube. The system is filled with air at 30°C temperature and at 76 cmHg pressure. Now the bulb of volume 3 L is immersed in water vapour at temperature 100°C while the other bulb is kept at 30°C.
Initially the total volume of the bulbs (V) = 3 + l= 4L,air pressure (p1) = 76 cmHg, temperature of air (T1) = 273 + 30 = 303 K
In the 2nd case, air pressure = p2
temperature of 3 L bulb = 100 + 273 = 373 K
temperature of 1L bulb = 30 + 273 = 303 K
We use the relation, n = \(\frac{p V}{R T}\)
In the 1st case, number of gram-molecules in the gas
= \(\frac{76 \times 3}{R \times 303}+\frac{76 \times 1}{R \times 303}=\frac{76}{R \times 303}(3+1)=\frac{76 \times 4}{R \times 303}\)
Let in the 2nd case, air pressure = p
No. of gram-molecules = \(\frac{p \times 3}{R \times 373}+\frac{p \times 1}{R \times 303}=\frac{p}{R}\left(\frac{3}{373}+\frac{1}{303}\right)\)
∴ No. of gram-molecules is unchanged,
= \(\frac{76 \times 4}{R \times 303}=\frac{p}{R}\left(\frac{3}{373}+\frac{1}{303}\right) \text { or, } \frac{76 \times 4}{303}=p\left(\frac{909+373}{373 \times 303}\right)\)
or, \(p=\frac{76 \times 4 \times 373}{1282}=88.4 \mathrm{cmHg} .\)
Derivation Of Pressure And Density Relation In Physics
Example 15. A narrow tube of uniform cross-section is closed at one end. Inside this tube a mercury thread of length hem detaches some air from the atmosphere outside. When the tube is held vertical keeping its closed end up, the length of the confined air column becomes l1 cm. Again the length of the air column becomes l2 cm when the tube is held vertical keeping its open end up. Find the magnitude of the atmospheric pressure.
Solution:
Given
A narrow tube of uniform cross-section is closed at one end. Inside this tube a mercury thread of length hem detaches some air from the atmosphere outside. When the tube is held vertical keeping its closed end up, the length of the confined air column becomes l1 cm. Again the length of the air column becomes l2 cm when the tube is held vertical keeping its open end up.
Let the atmospheric pressure =pcmHg; cross-section of the tube = α cm².
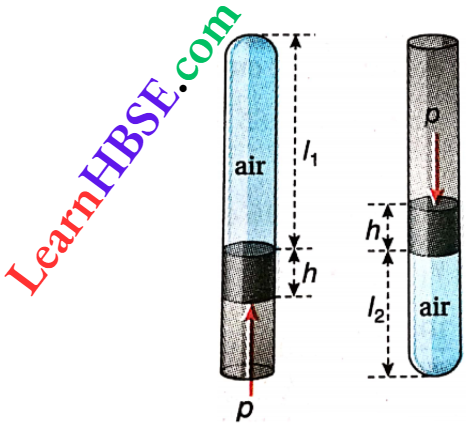
When the closed end of the tube is at the top, the volume of the confined air column V1 = l1αcm³;
pressure of confined air p1 = (p- h) cmHg.
Now when the open end is at the top the volume of the confined air column V2 = l2 α cm³
pressure of confined air p2 = (p + h) cmHg.
According to Boyle’s law p1V1 = p2 V2
or, \((p-h) l_1 \alpha=(p+h) l_2 \alpha\)
or, \((p-h) l_1=(p+h) l_2 or, p=\frac{l_1+l_2}{l_1-l_2} h\)
Hence, atmospheric pressure = \(\frac{l_1+l_2}{l_1-l_2}\) h cmHg.
Example 16. A glass tube of uniform area of cross-section and open at one end, encloses some air at 27°C by a 4 cm long mercury thread that acts like a piston. When the tube is held vertical with its open end up length of the air column in the tube is 9 cm. When the open end is held downwards by turning the tube, the length of the enclosed air column becomes 10 cm. Find
- The value of the atmospheric pressure,
- The temperature at which the length of the air column becomes 9 cm again, while the tube is still held inverted.
Solution:
Given
A glass tube of uniform area of cross-section and open at one end, encloses some air at 27°C by a 4 cm long mercury thread that acts like a piston. When the tube is held vertical with its open end up length of the air column in the tube is 9 cm. When the open end is held downwards by turning the tube, the length of the enclosed air column becomes 10 cm.
Let the atmospheric pressure = p cmHg, and the area of the cross-section of the tube = α cm².
When the open end is kept upwards, the volume of the confined air, V1 = 9αcm³ and pressure p1 = (p + 4)cmHg.
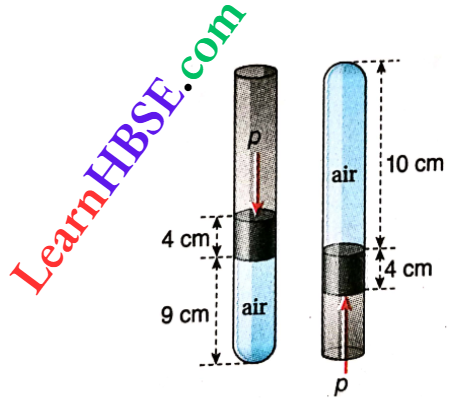
On inverting the tube, volume of the confined air V2 = 10α cm³ and pressure p2 =(p-4)cmHg.
Assuming temperature to be constant at 27°C, from Boyle’s law p1V1 = p2V2
or, 9α(p + 4) = 10α(p-4)
or, 9p + 36 = 10p-40, or, p= 76 cmHg
Suppose at T2 K, the length of the air column becomes 9 cm again when the tube is still held inverted.
Then its pressure, p2 = 76 – 4 = 72 cmHg and volume V2 = 9αcm³.
∴ \(\frac{p_1 V_1}{T_1}=\frac{p_2 V_2}{T_2}\)
or, \(\frac{(76+4) \times 9 \alpha}{273+27}=\frac{72 \times 9 \alpha}{T_2} \quad \text { or, } \frac{80}{300}=\frac{72}{T_2}\)
∴ \(T_2=270 \mathrm{~K}=(270-273)^{\circ} \mathrm{C}=-3^{\circ} \mathrm{C}\)
Pressure Density Temperature Relation In Thermodynamics
Example 17. A uniform glass tube closed at both ends, encloses air columns of equal lengths on either side of a mercury thread of length 5 cm, when the tube is placed horizontally. The pressure of the enclosed air is p. When the tube is placed at 60° to the vertical, the upper and the lower columns of air are of lengths 46 cm and 44.5 cm respectively. Find the value of p. The Temperature of the system remains constant at 30°C.
Solution:
Given
A uniform glass tube closed at both ends, encloses air columns of equal lengths on either side of a mercury thread of length 5 cm, when the tube is placed horizontally. The pressure of the enclosed air is p. When the tube is placed at 60° to the vertical, the upper and the lower columns of air are of lengths 46 cm and 44.5 cm respectively.
Let in the first case, length of air column on each side of the mercury thread = l.
Again, length of the tube = 44.5 + 46 + 5 = 95.5 cm.
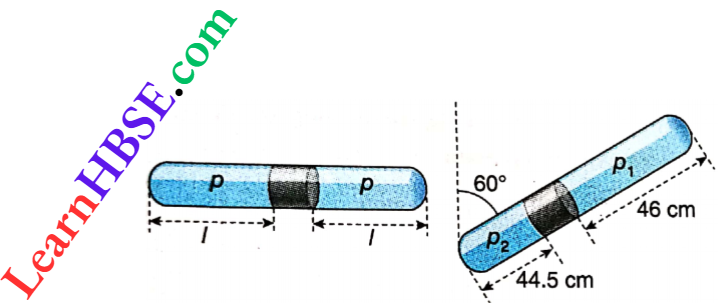
∴ \(l=\frac{95.5-5}{2}=45.25 \mathrm{~cm}\)
In the second case, p2 = p1 + 5cos60°
= p1+ 2 = (p1+ 2.5) cmHg
If in this case, V1 and V2 are the volumes of the upper and the lower air columns in the tube, then according to Boyle’s law,
p1V1 = p2V2
or, p1 · 46α’ = (p1 +2.5) x 44.5α
[where α = cross-sectional area of the tube]
Solving we get, p1= 74.17 cm
Again, pV = p1V1
or, \(p=\frac{p_1 V_1}{V}=\frac{74.17 \times 46 \alpha}{45.25 \alpha}=75.4 \mathrm{~cm}\)
i.e., p = 75.4 cmHg
Hydrostatic Pressure And Density Formula
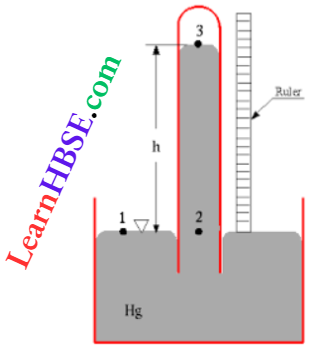
Example 18. The reading in a barometer changes from 75 cmHg to 25 cmHg when 10 cm³ of air at atmospheric pressure is introduced in the vacuum space of the barometer tube. What is the volume of air In the tube?
Solution:
Given
The reading in a barometer changes from 75 cmHg to 25 cmHg when 10 cm³ of air at atmospheric pressure is introduced in the vacuum space of the barometer tube.
Pressure of confined air =75-25 = 50 cmHg.
Initial volume of air V1 = 10 cm³ and initial pressure p1 = 75 cmHg.
Final volume of air = V2 cm³ and final pressure p2 = 50 cmHg.
Using Boyle’s law p1V1 = p2V2 we get,
⇒ \(V_2=\frac{p_1 V_1}{p_2}=\frac{75 \times 10}{50}=15\)
So, the volume occupied by the air inside the barometer is 15 cm³.
Example 19. The reading of a barometer decreases from 75 cmHg to 65 cmHg when some air is introduced in the vacuum space of the tube. The initial length of the space was 6 cm. If the area of the cross-section of the tube is 1 cm², what is the volume of this air at standard pressure?
Solution:
Given
The reading of a barometer decreases from 75 cmHg to 65 cmHg when some air is introduced in the vacuum space of the tube. The initial length of the space was 6 cm. If the area of the cross-section of the tube is 1 cm²
Standard pressure means p1 = 76 cmHg.
The volume of air at that pressure = V1 cm³.
Pressure of confined air p2 = 75 – 65 = 10 cmHg.
Volume of confined air V2 = {6+ (75-65)} x 1 = 16 cm³.
Using Boyle’s law, p1V1 = p2V2
or, \(V_1=\frac{p_2 V_2}{p_1}=\frac{10 \times 16}{76}=2.105\)
So the confined air occupies a volume of 2.105 cm³ at standard pressure.
Example 20. An air bubble of volume 20 cm³ forms in a lake at a depth of 40 m below the water surface. What will be its volume when it rises just below the water surface? (Standard atmospheric pressure = 76 cmHg)
Solution:
Given
An air bubble of volume 20 cm³ forms in a lake at a depth of 40 m below the water surface.
Volume of the air bubble at a depth of 40 cm, V1 = 20 cm³.
Pressure on the bubble there, p1 = atmospheric pressure + pressure due to 4000 cm of water column
= 76 x 13.6 x 980 + 4000 x 1 x 980
= (76 x 13.6 + 4000) x 980 dyn · cm-2
Let the volume of the bubble just below die surface be V2.
Pressure on the bubble just below the surface, p2 = 76 x 13.6 x 980 dyn • cm²
From Boyle’s law, p1V1 = p2V2
or, 980 x (76 x 13.6 + 4000) x 20 = V2 x 76 x 13.6 x 980
∴ \(V_2=\frac{(76 \times 13.6+4000) \times 20}{76 \times 13.6}=97.4 \mathrm{~cm}^3\)
Ideal Gas Law Relation Between Pressure And Density
Example 21. In a capillary tube, closed at one end, some air is enclosed by a mercury thread of length 10 cm. When the tube is kept horizontal the length of the air column is 17 cm. When it is held vertical with the open end up, the length changes to 15 cm. What will be the length of the air column when the tube is held vertical with the open end downwards?
Solution:
Given
In a capillary tube, closed at one end, some air is enclosed by a mercury thread of length 10 cm. When the tube is kept horizontal the length of the air column is 17 cm. When it is held vertical with the open end up, the length changes to 15 cm.
Let atmospheric pressure = pcmHg; crosssectional area of the tube = α cm².
When the tube is horizontal, pressure of the confined air, p1 = p cmHg,
volume of this confined air, V1 = 17 α cm³.
When the tube is held vertical with the open end up, pressure of the confined air, p2 = (p + 10) cmHg,
volume of this confined air, V2 = 15α cm³
According to Boyle’s law, px 17α = (p + 10) x 15α or, p = 75
∴ Atmospheric pressure = 75 cmHg
Again, when the tube is held vertical with the open end downwards,
pressure of the confined air, p3 = (p-10) cmHg,
volume of this confined air, V3 = hα cm³
[where h = length of the air column]
According to Boyle’s law, \(p_1 V_1=p_3 V_3 \text { or, } p \times 17 \alpha=(p-10) \times h \alpha \)
or, h = \(\frac{17 p}{p-10}=\frac{17 \times 75}{65}=19.6 \mathrm{~cm}\).
Example 22. Volume of a room is 15 m x 12 m x 8 m. The room was at 22°C in the morning. What is the percentage of initial volume of air of the room that Is expelled when the room temperature reaches 30° C at noon? The pressure remains constant during die change of temperature.
Solution:
Given
Volume of a room is 15 m x 12 m x 8 m. The room was at 22°C in the morning.
From Charles’ law: \(\frac{V_1}{T_1}=\frac{V_2}{T_2}\) or, \(\frac{V_1}{V_2}=\frac{T_1}{T_2}\)
or, \(\frac{V_1}{V_2-V_1}=\frac{T_1}{T_2-T_1} or, \frac{V_2-V_1}{V_1} \times 100=\frac{T_2-T_1}{T_1} \times 100\)
Given, \(T_1=22^{\circ} \mathrm{C}=295 \mathrm{~K}, T_2=30^{\circ} \mathrm{C}=303 \mathrm{~K}, \quad V_2-V_1\) = volume of expelled air
∴ \(\frac{V_2-V_1}{V_1} \times 100=\frac{T_2-T_1}{T_1} \times 100=\frac{303-295}{295} \times 100=2.7\)
∴ 2.7% of air will be expelled from the room.
Example 23. Air is enclosed in a glass container at 67°C. Find the temperature to which the container is to be raised at constant pressure, so that 1/3 rd of the final volume of the air is expelled from the vessel? (Neglect the expansion of glass)
Solution:
Given
Air is enclosed in a glass container at 67°C.
Let initially the volume of air = V1
In this case, temperature of air, T1 = 273 + 67 = 340 K
Suppose the volume of the air becomes V2 at a temperature T2.
∴ Volume of expelled air = 1/3 V2
∴ \(V_2=V_1+\frac{1}{3} V_2 \text { or, } V_2=\frac{3}{2} V_1\)
Since the pressure is constant, we can write \(\frac{V_1}{T_1}=\frac{V_2}{T_2}\)
∴ \(\frac{V_1}{340}=\frac{\frac{3}{2} V_1}{T_2}\)
or, \(T_2=\frac{3}{2} \times 340=510 \mathrm{~K}=(510-273)^{\circ} \mathrm{C}=237^{\circ} \mathrm{C} .\)